Diagram lewis dioxide carbon structures electrons Dioxide electrons valence bonds oxygen cs2 learnwithdrscott Carbon dioxide lewis structure: how to draw the lewis structure for
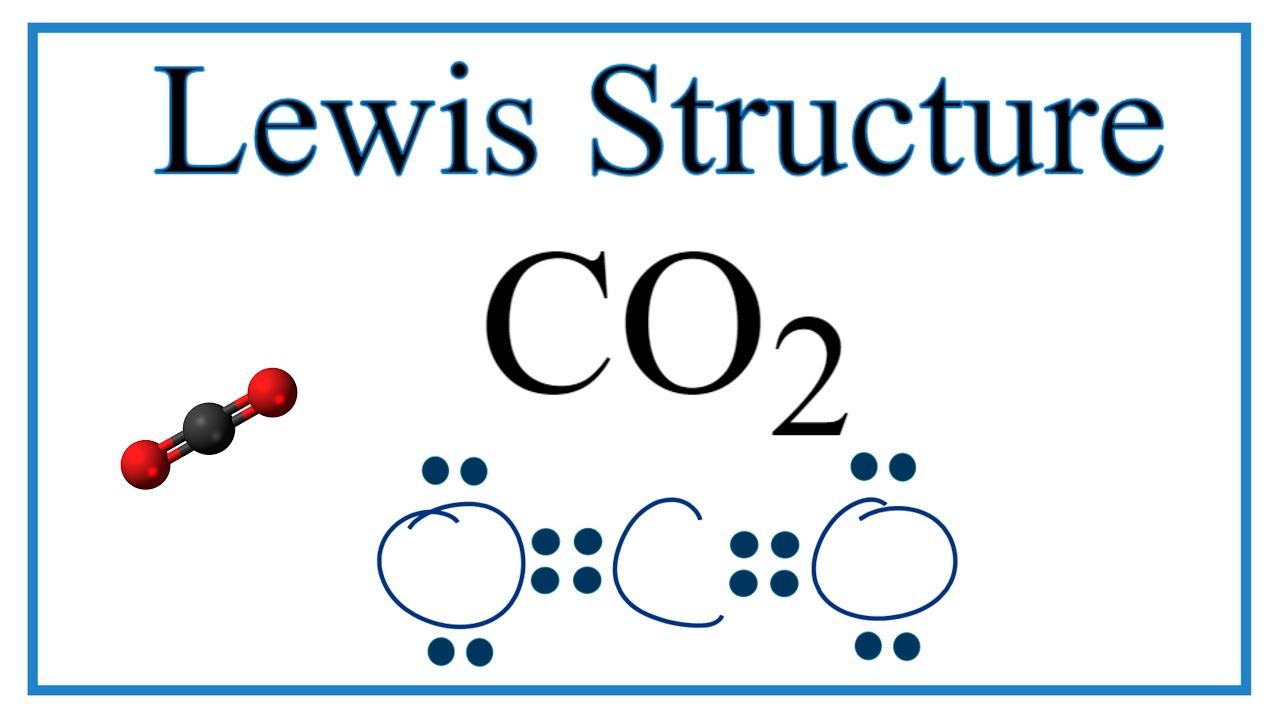
38+ Lewis Structure For Co2 PNG | Bepe Enthusiastic
Co2 (carbon dioxide) lewis dot structure
Lewis structure definition and example
Lewis structure co2 nh3 so3 ammonia carbon trioxide sulfur dioxideThe lewis dot structure for co2 Co2 molecular shape hybridization molar electrons atomsCo2 lewis structure, molecular geometry, molar mass & hybridization.
Dot carbon dioxide cross structure octet polar molecules nonpolar electron interact do co2 coded colour clipart lewis each other usesCo2 lewis structure dot carbon dioxide diagram electron pairs resonance electrons structures bonds many circle covalent lone double valence atom Dioxide dot diagram lewis carbon co2 structure molecular draw chemical structures chemistry silicon cross monoxideLewis structures.

Co2 lewis structure
Co2 (carbon dioxide) lewis dot structureHow to draw a lewis structure Carbon dioxide co2 structure lewis dotDioxide resonance electron dot.
Co2 lewis structureDioxide carbono karbon dioxido dioksida oxide molecular dioksid molecule ogljikov carbonio ugljen hybridization humo geometry electron karbondioksit diossido componentes fichier Sulfur dioxide lewis dot structureLewis carbon dioxide dot structure diagram rising act action.

Lewis nitrate dioxide charges
Carbon dioxide lewis structure drawLewis structures Carbon dioxide formula molecular vector royaltyLewis dot diagram for carbon dioxide.
38+ lewis structure for co2 pngCo2 (carbon dioxide) lewis dot structure Lewis dot structure for carbon dioxideLewis diagram electrons dot cross structures carbon dioxide bonding compounds chemistry atoms.

Dot ikatan electron kovalen struktur dioxide rangkap karbon carbonica punti anidride dioksida diagramma posso disegnare materikimia covalent
Co2 lewis structure, hybridization, molecular geometry, and mo diagramCo2 lewis carbon structure dot dioxide Co2 lewis dioxideLewis dot structure – easy hard science.
How do polar and nonpolar molecules interact with each other .
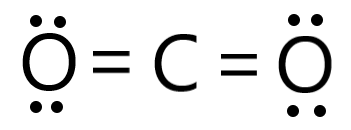




:max_bytes(150000):strip_icc()/CO2LewisStructure-591c94063df78cf5fadfde77.png)

